Stanford researchers have been working on gene therapies for epidermolysis bullosa, or “butterfly disease,” for over a decade. A new gel helped wounds heal and stay healed in a clinical trial.
A gene therapy gel applied to the wounds of nine people — three of whom were children — with the blistering skin disease epidermolysis bullosa helped the wounds heal and remain healed for several months in a trial headed by researchers at Stanford Medicine.
The trial is the first to show that gene therapy vectors for skin diseases can be effective when applied topically. It is also the first trial of gene therapy in children with epidermolysis bullosa.
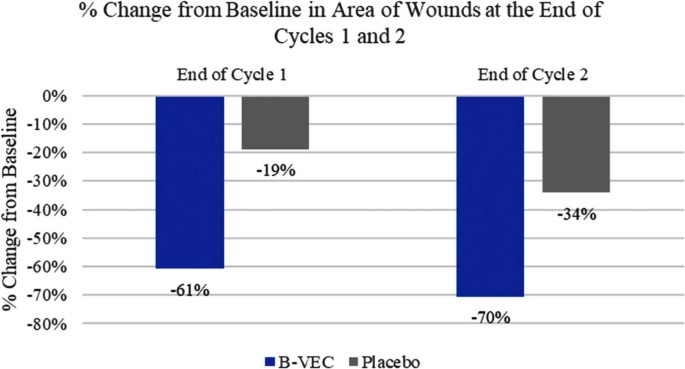
A large 10-year-old wound covering most of the side of a patient’s body showed 70% healing, while all other wounds closed completely with treatment. For one patient, a chronic wound that had persisted for five years closed completely after two successive rounds of treatments and remained closed throughout eight months of monitoring.
“The wounds heal quickly but, even more importantly, they stay closed,” said Peter Marinkovich, MD, director of Stanford Medicine’s Blistering Disease Clinic. “The therapy strengthens the skin and breaks the painful and destructive cycle of wound opening and closing that patients with epidermolysis bullosa experience.”
The gel is stable at room temperature and can be applied without specialized expertise during routine bandage changes — key advantages for patients worldwide who have difficulty accessing specialized medical care, Marinkovich said.
Marinkovich, an associate professor of dermatology, is the senior author of the study, published online March 28 in Nature Medicine. Former research scientist Irina Gurevich, PhD, is the lead author. The study was funded by Krystal Biotech Inc., which is testing and developing the gel for clinical use.
‘Butterfly children’
The nine patients in the trial had a form of epidermolysis bullosa called recessive dystrophic epidermolysis bullosa, or RDEB. People with RDEB have mutations in a gene that encodes a protein called collagen VII. Collagen VII binds the outer and middle layers of the skin together. Without this protein, the layers slide across each other, causing blisters that progress to severely painful open wounds.
People with the disease are called “butterfly children” because their skin is so fragile that the slightest touch can cause it to blister. They are also susceptible to wound infections and skin cancer, and often die in early adulthood.
For more than a decade, researchers at Stanford Medicine have been developing therapies designed to deliver an intact copy of the gene, called COL7A1, to RDEB patients. In 2016, Marinkovich and his colleagues announced the results of a trial using genetically corrected patient skin grafts expressing COL7A1. They found that the approach was safe and that it helped wound healing. But engineering and growing the grafts was laborious. They also had to be surgically applied while the patients were under general anesthesia, and follow-up required a week of hospitalization.
In contrast, the gel used in the current study is topically applied in short, weekly outpatient clinic visits during regular bandage changes. The gel delivers a modified herpes simplex virus that has been genetically engineered so that it cannot replicate or spread to other areas of the body. The virus delivers two copies of the COL7A1 directly to the surface of the wound.
Unlike many other viruses used for gene therapy, the herpes simplex virus does not integrate into its host’s genome when it infects a cell. This is an advantage because there is a slight risk that integration can disrupt normal gene expression and cause cancer.
Before launching a clinical trial with the nine RDEB patients, including three children, the researchers tested the therapy in RDEB skin cells grown in a lab, in skin grafts from patients and in mice genetically engineered to have the disease to confirm that it led to the expression of collagen VII in the cells.
Testing the gel in patients
The researchers treated two wounds on each patient by applying the gel to one wound and a placebo to the other over a 25-day period. After three months, they assessed the wounds to determine how well they had healed, then monitored them for several more weeks to see if they reopened.
Most wounds treated with the gene therapy gel closed within three months after treatment ended. The exception was a wound one participant had on a foot for five years, but that wound healed after a second 25-day treatment cycle and remained healed for eight months. In contrast, the wounds treated with the placebo healed and re-blistered at variable rates during the trial.
Biopsies of the treated skin from seven of the trial patients showed that it was making collagen VII as early as nine days after the initiation of treatment. In at least one case, the expression persisted for nearly 100 days. Trial participants experienced few adverse events, and those that arose were mild.
“We saw no problems with repeated administration of the gel, and the patients and their families were very enthusiastic about the results,” Marinkovich said. “I’m excited that, if approved for clinical use by the Food and Drug Administration, we will be able to reach many more patients with this devastating disease.”
A phase 3 trial of the therapy has been completed. The researchers plan to publish the results and submit them to the FDA for approval of the drug.
The study was funded by Pittsburgh-based Krystal Biotech Inc. and the Palo Alto Veterans Affairs Medical Center. Marinkovich is also an investigator for four companies studying molecular corrective therapies for RDEB: Castle Creek Pharmaceuticals Inc., Abeona Therapeutics Inc., WINGS Therapeutics Inc. and Phoenix Tissue Repair Inc.

Leave a Reply