
How do vaccines work?
Germs are all around us, both in our environment and in our bodies. When a person is susceptible and they encounter a harmful organism, it can lead to disease and death.
The body has many ways of defending itself against pathogens (disease-causing organisms). Skin, mucus, and cilia (microscopic hairs that move debris away from the lungs) all work as physical barriers to prevent pathogens from entering the body in the first place.
When a pathogen does infect the body, our body’s defences, called the immune system, are triggered and the pathogen is attacked and destroyed or overcome.
The body’s natural response
A pathogen is a bacterium, virus, parasite or fungus that can cause disease within the body. Each pathogen is made up of several subparts, usually unique to that specific pathogen and the disease it causes. The subpart of a pathogen that causes the formation of antibodies is called an antigen. The antibodies produced in response to the pathogen’s antigen are an important part of the immune system. You can consider antibodies as the soldiers in your body’s defense system. Each antibody, or soldier, in our system is trained to recognize one specific antigen. We have thousands of different antibodies in our bodies. When the human body is exposed to an antigen for the first time, it takes time for the immune system to respond and produce antibodies specific to that antigen.
In the meantime, the person is susceptible to becoming ill.
Once the antigen-specific antibodies are produced, they work with the rest of the immune system to destroy the pathogen and stop the disease. Antibodies to one pathogen generally don’t protect against another pathogen except when two pathogens are very similar to each other, like cousins. Once the body produces antibodies in its primary response to an antigen, it also creates antibody-producing memory cells, which remain alive even after the pathogen is defeated by the antibodies. If the body is exposed to the same pathogen more than once, the antibody response is much faster and more effective than the first time around because the memory cells are at the ready to pump out antibodies against that antigen.
This means that if the person is exposed to the dangerous pathogen in the future, their immune system will be able to respond immediately, protecting against disease.

How vaccines help
Vaccines contain weakened or inactive parts of a particular organism (antigen) that triggers an immune response within the body. Newer vaccines contain the blueprint for producing antigens rather than the antigen itself. Regardless of whether the vaccine is made up of the antigen itself or the blueprint so that the body will produce the antigen, this weakened version will not cause the disease in the person receiving the vaccine, but it will prompt their immune system to respond much as it would have on its first reaction to the actual pathogen.

Some vaccines require multiple doses, given weeks or months apart. This is sometimes needed to allow for the production of long-lived antibodies and development of memory cells. In this way, the body is trained to fight the specific disease-causing organism, building up memory of the pathogen so as to rapidly fight it if and when exposed in the future.
Herd immunity
When someone is vaccinated, they are very likely to be protected against the targeted disease. But not everyone can be vaccinated. People with underlying health conditions that weaken their immune systems (such as cancer or HIV) or who have severe allergies to some vaccine components may not be able to get vaccinated with certain vaccines. These people can still be protected if they live in and amongst others who are vaccinated. When a lot of people in a community are vaccinated the pathogen has a hard time circulating because most of the people it encounters are immune. So the more that others are vaccinated, the less likely people who are unable to be protected by vaccines are at risk of even being exposed to the harmful pathogens. This is called herd immunity.
This is especially important for those people who not only can’t be vaccinated but may be more susceptible to the diseases we vaccinate against. No single vaccine provides 100% protection, and herd immunity does not provide full protection to those who cannot safely be vaccinated. But with herd immunity, these people will have substantial protection, thanks to those around them being vaccinated.
Vaccinating not only protects yourself, but also protects those in the community who are unable to be vaccinated. If you are able to, get vaccinated.

How are vaccines developed?
What are the ingredients in a vaccine?
Vaccines contain tiny fragments of the disease-causing organism or the blueprints for making the tiny fragments. They also contain other ingredients to keep the vaccine safe and effective. These latter ingredients are included in most vaccines and have been used for decades in billions of doses of vaccine.
Each vaccine component serves a specific purpose, and each ingredient is tested in the manufacturing process. All ingredients are tested for safety.
Antigen
All vaccines contain an active component (the antigen) which generates an immune response, or the blueprint for making the active component. The antigen may be a small part of the disease-causing organism, like a protein or sugar, or it may be the whole organism in a weakened or inactive form.

Preservatives
Preservatives prevent the vaccine from becoming contaminated once the vial has been opened, if it will be used for vaccinating more than one person. Some vaccines don’t have preservatives because they are stored in one-dose vials and are discarded after the single dose is administered. The most commonly used preservative is 2-phenoxyethanol. It has been used for many years in a number of vaccines, is used in a range of baby care products and is safe for use in vaccines, as it has little toxicity in humans.
Stabilizers
Stabilizers prevent chemical reactions from occurring within the vaccine and keep the vaccine components from sticking to the vaccine vial.
Stabilizers can be sugars (lactose, sucrose), amino acids (glycine), gelatin, and proteins (recombinant human albumin, derived from yeast).

Surfactants
Surfactants keep all the ingredients in the vaccine blended together. They prevent settling and clumping of elements that are in the liquid form of the vaccine. They are also often used in foods like ice cream.
Residuals
Residuals are tiny amounts of various substances used during manufacturing or production of vaccines that are not active ingredients in the completed vaccine. Substances will vary depending on the manufacturing process used and may include egg proteins, yeast or antibiotics. Residual traces of these substances which may be present in a vaccine are in such small quantities that they need to be measured as parts per million or parts per billion.
Diluent
A diluent is a liquid used to dilute a vaccine to the correct concentration immediately prior to use. The most commonly used diluent is sterile water.
Adjuvant
Some vaccines also contain adjuvants. An adjuvant improves the immune response to the vaccine, sometimes by keeping the vaccine at the injection site for a little longer or by stimulating local immune cells.
The adjuvant may be a tiny amount of aluminium salts (like aluminium phosphate, aluminium hydroxide or potassium aluminium sulphate). Aluminium has been shown not to cause any long-term health problems, and humans ingest aluminium regularly through eating and drinking.
How are vaccines developed?
Most vaccines have been in use for decades, with millions of people receiving them safely every year. As with all medicines, every vaccine must go through extensive and rigorous testing to ensure it is safe before it can be introduced in a country’s vaccine programme.
Each vaccine under development must first undergo screenings and evaluations to determine which antigen should be used to invoke an immune response. This preclinical phase is done without testing on humans. An experimental vaccine is first tested in animals to evaluate its safety and potential to prevent disease.
If the vaccine triggers an immune response, it is then tested in human clinical trials in three phases.
Phase 1
The vaccine is given to a small number of volunteers to assess its safety, confirm it generates an immune response, and determine the right dosage. Generally in this phase vaccines are tested in young, healthy adult volunteers.
Phase 2
The vaccine is then given to several hundred volunteers to further assess its safety and ability to generate an immune response. Participants in this phase have the same characteristics (such as age, sex) as the people for whom the vaccine is intended. There are usually multiple trials in this phase to evaluate various age groups and different formulations of the vaccine. A group that did not get the vaccine is usually included in phase as a comparator group to determine whether the changes in the vaccinated group are attributed to the vaccine, or have happened by chance.
Phase 3
The vaccine is next given to thousands of volunteers – and compared to a similar group of people who didn’t get the vaccine, but received a comparator product – to determine if the vaccine is effective against the disease it is designed to protect against and to study its safety in a much larger group of people. Most of the time phase three trials are conducted across multiple countries and multiple sites within a country to assure the findings of the vaccine performance apply to many different populations.
During phase two and phase three trials, the volunteers and the scientists conducting the study are shielded from knowing which volunteers had received the vaccine being tested or the comparator product. This is called “blinding” and is necessary to assure that neither the volunteers nor the scientists are influenced in their assessment of safety or effectiveness by knowing who got which product. After the trial is over and all the results are finalized, the volunteers and the trial scientists are informed who received the vaccine and who received the comparator.
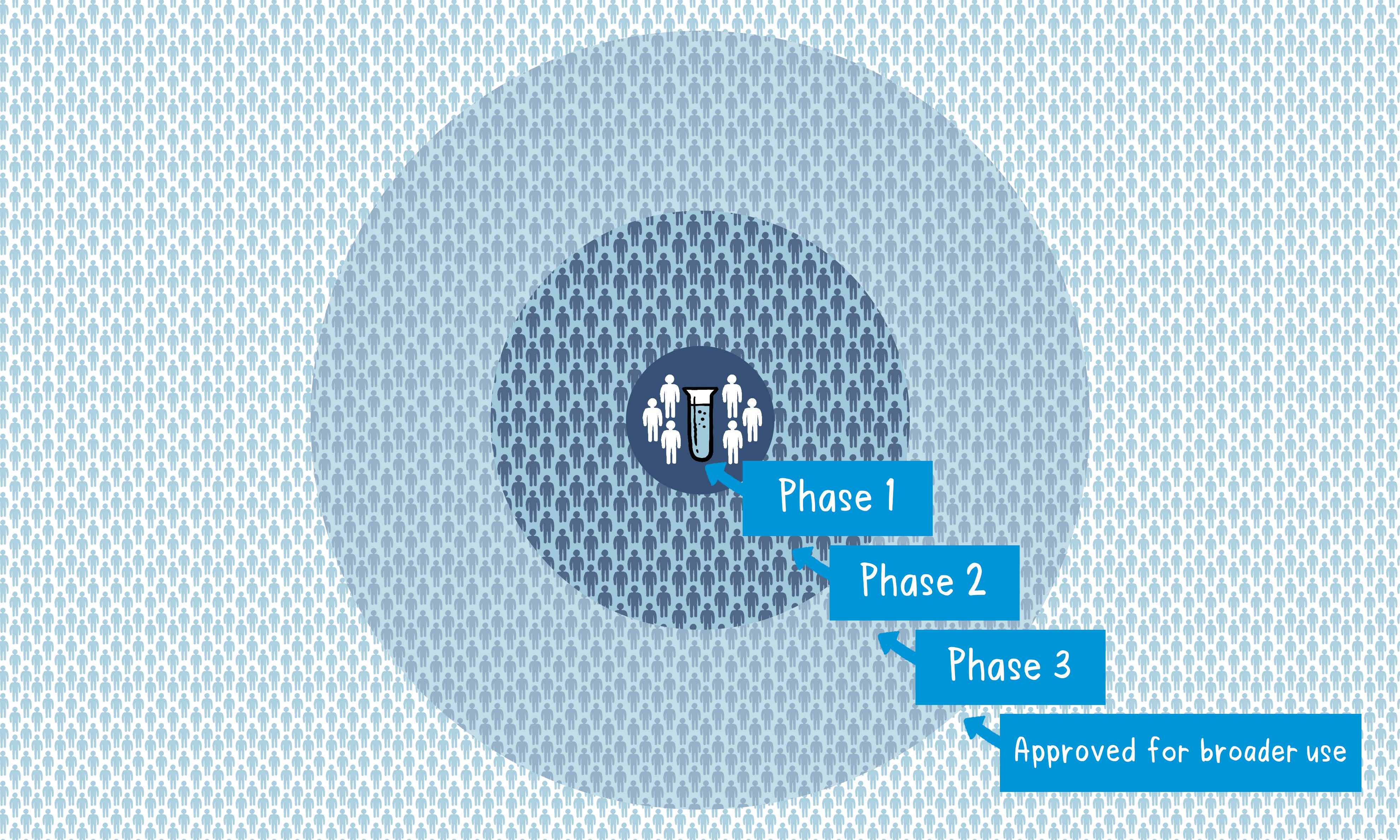
When the results of all these clinical trials are available, a series of steps is required, including reviews of efficacy and safety for regulatory and public health policy approvals. Officials in each country closely review the study data and decide whether to authorize the vaccine for use. A vaccine must be proven to be safe and effective across a broad population before it will be approved and introduced into a national immunization programme. The bar for vaccine safety and efficacy is extremely high, recognizing that vaccines are given to people who are otherwise healthy and specifically free from the illness.
Further monitoring takes place in an ongoing way after the vaccine is introduced. There are systems to monitor the safety and effectiveness of all vaccines. This enables scientists to keep track of vaccine impact and safety even as they are used in a large number of people, over a long time frame. These data are used to adjust the policies for vaccine use to optimize their impact, and they also allow the vaccine to be safely tracked throughout its use.
Once a vaccine is in use, it must be continuously monitored to make sure it continues to be safe.
The different types of COVID-19 vaccines
As of December 2020, there are over 200 vaccine candidates for COVID-19 being developed. Of these, at least 52 candidate vaccines are in human trials. There are several others currently in phase I/II, which will enter phase III in the coming months (for more information on the clinical trial phases, see part three of our Vaccine Explained series).
Why are there so many vaccines in development?
Typically, many vaccine candidates will be evaluated before any are found to be both safe and effective. For example, of all the vaccines that are studied in the lab and laboratory animals, roughly 7 out of every 100 will be considered good enough to move into clinical trials in humans. Of the vaccines that do make it to clinical trials, just one in five is successful. Having lots of different vaccines in development increases the chances that there will be one or more successful vaccines that will be shown to be safe and efficacious for the intended prioritized populations.

The different types of vaccines
There are three main approaches to designing a vaccine. Their differences lie in whether they use a whole virus or bacterium; just the parts of the germ that triggers the immune system; or just the genetic material that provides the instructions for making specific proteins and not the whole virus.
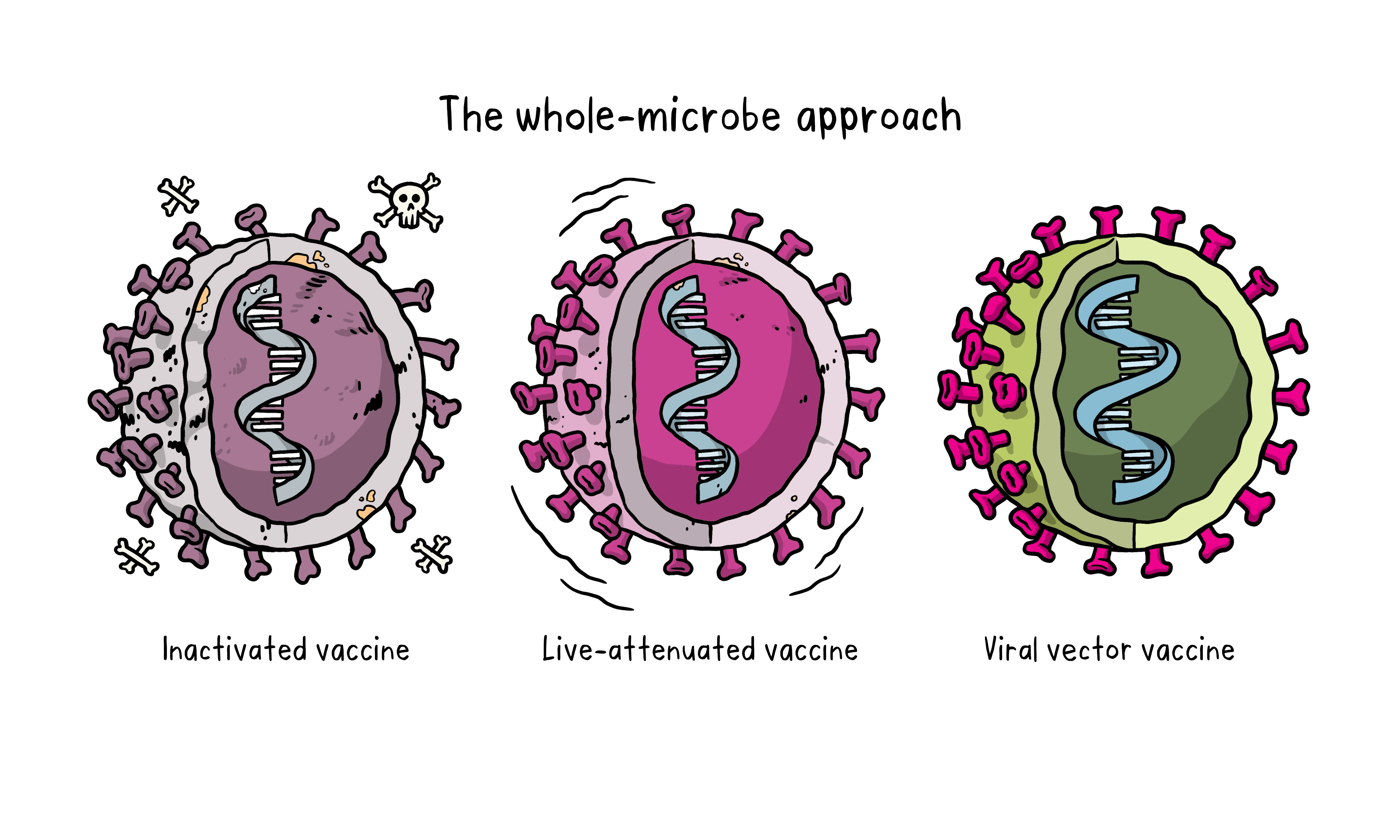
The whole-microbe approach
Inactivated vaccine
The first way to make a vaccine is to take the disease-carrying virus or bacterium, or one very similar to it, and inactivate or kill it using chemicals, heat or radiation. This approach uses technology that’s been proven to work in people – this is the way the flu and polio vaccines are made – and vaccines can be manufactured on a reasonable scale.
However, it requires special laboratory facilities to grow the virus or bacterium safely, can have a relatively long production time, and will likely require two or three doses to be administered.
Live-attenuated vaccine
A live-attenuated vaccine uses a living but weakened version of the virus or one that’s very similar. The measles, mumps and rubella (MMR) vaccine and the chickenpox and shingles vaccine are examples of this type of vaccine. This approach uses similar technology to the inactivated vaccine and can be manufactured at scale. However, vaccines like this may not be suitable for people with compromised immune systems.
Viral vector vaccine
This type of vaccine uses a safe virus to deliver specific sub-parts – called proteins – of the germ of interest so that it can trigger an immune response without causing disease. To do this, the instructions for making particular parts of the pathogen of interest are inserted into a safe virus. The safe virus then serves as a platform or vector to deliver the protein into the body. The protein triggers the immune response. The Ebola vaccine is a viral vector vaccine and this type can be developed rapidly.
The subunit approach

A subunit vaccine is one that only uses the very specific parts (the subunits) of a virus or bacterium that the immune system needs to recognize. It doesn’t contain the whole microbe or use a safe virus as a vector. The subunits may be proteins or sugars. Most of the vaccines on the childhood schedule are subunit vaccines, protecting people from diseases such as whooping cough, tetanus, diphtheria and meningococcal meningitis.
The genetic approach (nucleic acid vaccine)
Unlike vaccine approaches that use either a weakened or dead whole microbe or parts of one, a nucleic acid vaccine just uses a section of genetic material that provides the instructions for specific proteins, not the whole microbe. DNA and RNA are the instructions our cells use to make proteins. In our cells, DNA is first turned into messenger RNA, which is then used as the blueprint to make specific proteins.

A nucleic acid vaccine delivers a specific set of instructions to our cells, either as DNA or mRNA, for them to make the specific protein that we want our immune system to recognize and respond to.
The nucleic acid approach is a new way of developing vaccines. Before the COVID-19 pandemic, none had yet been through the full approvals process for use in humans, though some DNA vaccines, including for particular cancers, were undergoing human trials. Because of the pandemic, research in this area has progressed very fast and some mRNA vaccines for COVID-19 are getting emergency use authorization, which means they can now be given to people beyond using them only in clinical trials.
Source: The WHO


Leave a Reply